What Determines the Color of Something?
Light and Color
A red apple does not emit red light, it just reflects red light. When white light hits an object, said object absorbs some wavelengths and reflects others (remember that white light is made up of all the colors of wavelengths). The reflected wavelengths determine the color of the object since those are the ones that hit our eyes.
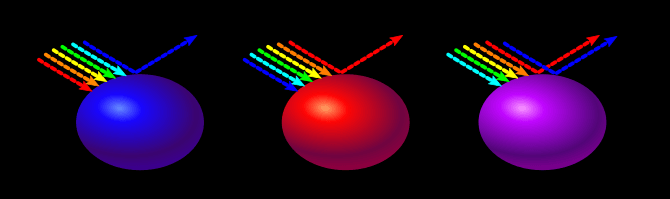
A red object absorbs all wavelengths except red. A purple object absorbs all wavelengths except red and blue.
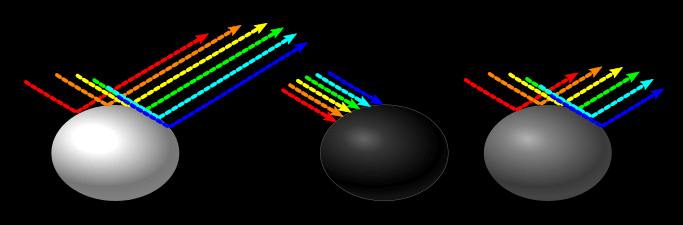
A white object reflects all wavelengths of light. A black object absorbs all wavelengths of light. Now not all of the light is absorbed/reflected, otherwise white objects would blind us, and black objects would appear to have no dimension. It is more the ratio of the different wavelengths (colors) that are absorbed/reflected that determine the color. Grey reflects more light than black, but not as much as white.
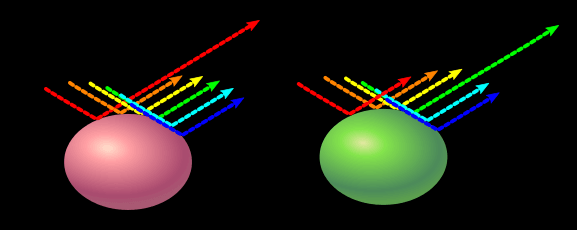
Pastel colors reflect all wavelengths of light like white, but they have one color they reflect more than the others, which gives it its hue.
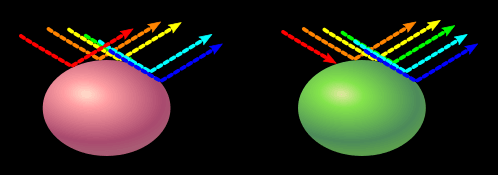
Pastel colors can also be made by reflecting all colors except one. Because it doesn't reflect this color, it be won't white, but instead the opposite of the color it absorbs. Without green to balance red out, the object will be pink. Without red to balance green out, the object will be pastel green. There are infact many ways to make the same color, but that will be explored more on a later page (that is yet to be made).
Chemical Structure and Color
So what determines what color something is? Why is yellow paint yellow? There are a few different theories on the chemical causes of color, but all involve the specific amount of energy an atom, compound, or crystal structure needs to reach a higher energy state.Causes of Color They are the most stable at ground state, their lowest energy state, but they have higher energy states they can be at. These states are at very specific energies which are different for each molecule.
Light is a form of energy, thus it can give said energy to the molecules. Most important is the fact that these molecules can only reach a higher energy state if the energy it receives is exactly the same as the difference between a higher energy state and the initial. Each color of light has a different amount of energy. If the energy of a color of light matches that of the energy needed to excite the molecule to a higher energy state, said molecule will absorb that color. The rest of the colors will be reflected off of the molecule. Atoms and Light
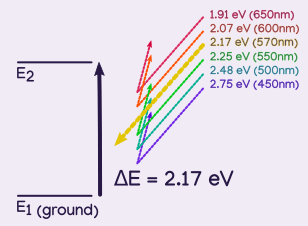
Suppose it takes 2.17 eV (electron volts, a unit of energy) to move a molecule from ground state to its first excited state. Each wavelength carries a different amount of energy, but only yellow at 570nm has exactly 2.17eV, so it is the only wavelength that gets absorbed by the molecule, the other wavelengths are relfected.
With all reflected except for yellow, this molecule would be a pastel purple (since purple is the opposite of yellow, and yellow was the least reflected).
So if a chemical can only absorb one specific energy, and it reflects all the other wavelengths, wouldn't all objects be pastel? How is something deep red if so many other wavelengths are being reflected?
Keep in mind that most things are composed of many different chemicals, and the chemicals themselves may be complex with different shaped sections that absorb different colors. So while some structures may reflect blue light, another structure in the same object will absorb the blue light.
Pigments and Light
We know that mixtures of light results in a lighter color as it gets closer to white light, but pigments get darker when mixed, why?
The reason a mixture of pigments results in less color is because the different chemical structures that absorb wavelengths are layered, meaning more light is absorbed, and less is reflected. Consider blue and orange paint mixed together. The chemical structures making blue and the ones making orange are still intact and distinct, simply in tight proximity. Thus, while the blue paint would normally reflect the blue wavelengths, the orange pigment absorbs some of them, likewise the blue pigment absorbs some of the orange wavelengths normally reflected by the orange, resulting in less light overall being reflected. Whether this mixture results in brown or a warm dull blue depends on the ratio of the colors.
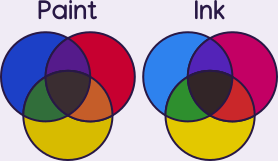
This is called the subtractive color model, which means at each additional pigment, the darker the resultant color gets. Ideally an equal mix of primary colors will create black. The more transparent the material is, the truer to reality this is. For that reason, transparent dyes can approximate black better than opaque pigments. Light follows the additive color model instead. Read more about primary colors.
Colored Light on Objects
So we know what happens when white light hits an object, but what happens when only red light hits an object? What about a mix of red and blue (purple)? The same logic applies, we just have to keep in mind the available wavelengths we have to absorb and reflect.
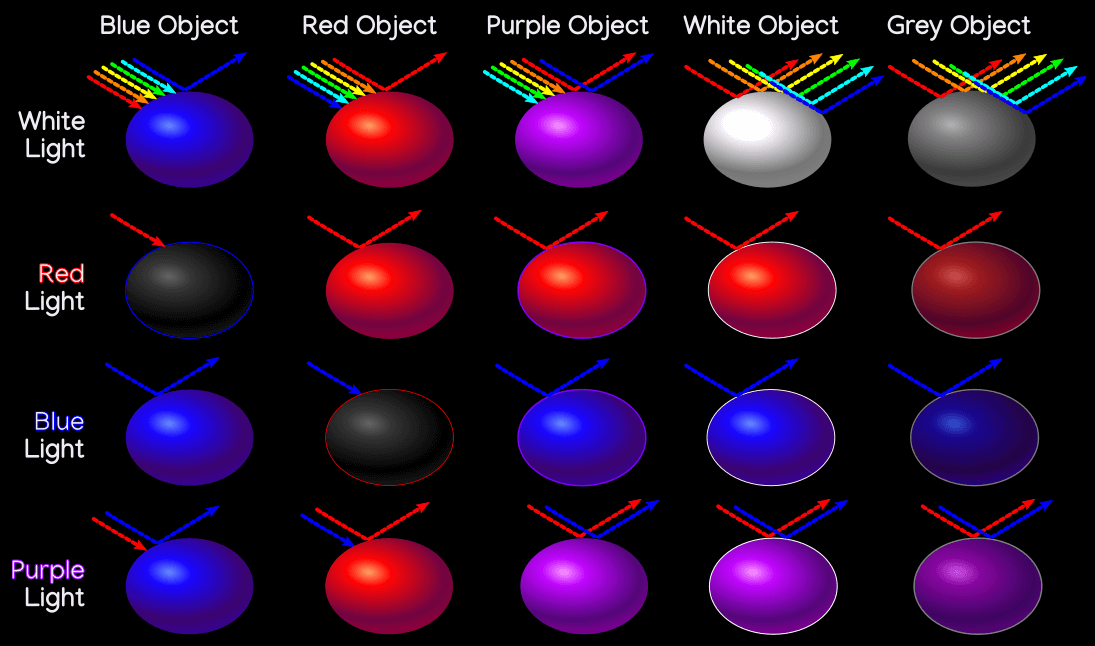
Take some time to study this diagram. It shows five objects, one blue, one red, one purple, one white, and one grey, under four different lighting conditions: white, red, blue, and purple light. Under white light they all appear as expected.
Under red light, the red object is able to reflect red light as normal, but the blue object absorbs the red light, and with no blue light to reflect, it is black. Similarly the blue object reflects blue light as normal under blue light, but the red object appears black since it has no red light to reflect.
Under purple light the purple object appears as normal, and interestingly, so do the red and blue objects! Since purple light is made up of blue and red wavelengths, the red object has red light to reflect, and the blue object has blue light to reflect. Under red light, the purple object has no blue to reflect, so it appears red; the same happens with blue light.
White objects normally reflect all wavelengths of light, but if it only has one wavelength to reflect it will appear that color, hence why the white object is red under red light, blue under blue light, and purple under purple light. Grey behaves similarly, but since it reflects less light overall, the colors will be dimmer.
Suggested Next Pages: Primary Colors Prisms and the Rainbow